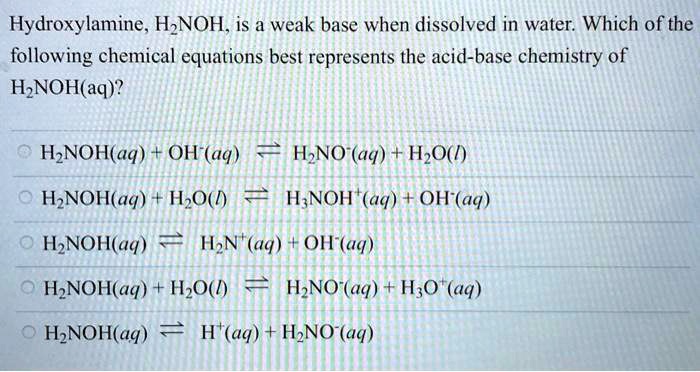Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com

Oxides are more acidic than hydroxylamine `(NH_2 OH)`. Conjugate base of oxime is resonance stabilised. - Sarthaks eConnect | Largest Online Education Community

Reaction of dC and 5MedC with hydroxylamine derivatives. Depiction of... | Download Scientific Diagram
Hydroxylamine sulfate (Hydroxylammonium sulfate) 99,999 % base métaux-traces - Materiel pour Laboratoire

The Hydroxylamine Reaction of Sensory Rhodopsin II: Light-Induced Conformational Alterations with C13C14 Nonisomerizable Pigment: Biophysical Journal
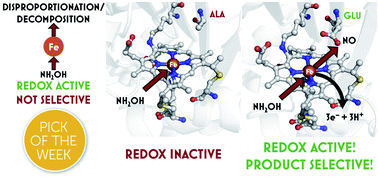
Controlling a burn: outer-sphere gating of hydroxylamine oxidation by a distal base in cytochrome P460 - Chemical Science (RSC Publishing)

An Integrated Process for the Synthesis of Solid Hydroxylamine Salt with Ammonia and Hydrogen Peroxide as Raw Materials | Industrial & Engineering Chemistry Research

Formation of Aromatic Amidoximes with Hydroxylamine using Microreactor Technology | Organic Process Research & Development

One‐Pot Synthesis of Hydroxamic Acids from Aldehydes and Hydroxylamine - Dettori - 2014 - Advanced Synthesis & Catalysis - Wiley Online Library
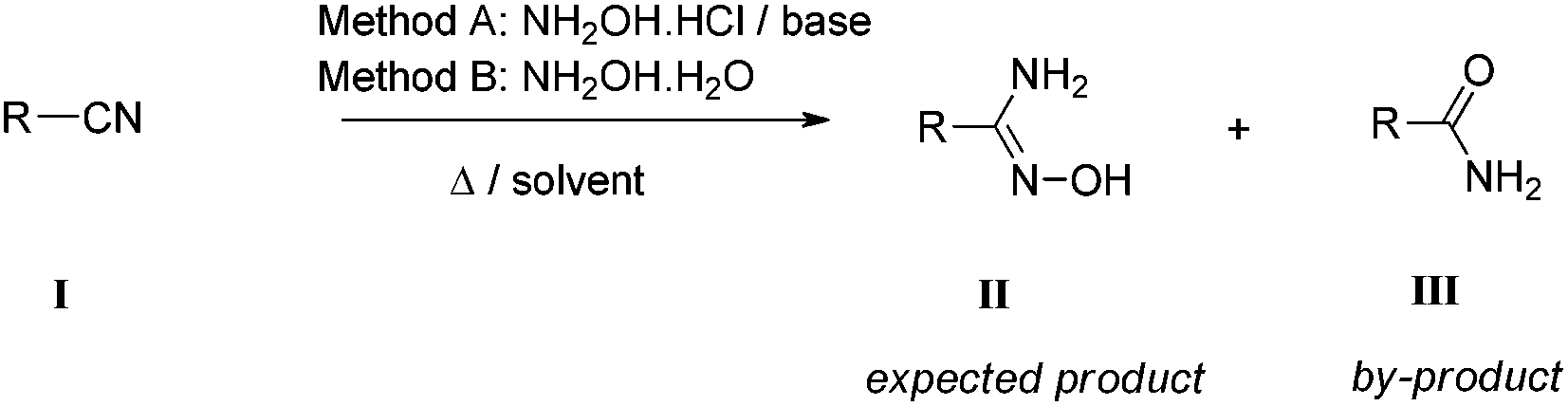
An experimental and theoretical study of reaction mechanisms between nitriles and hydroxylamine - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C4OB00854E
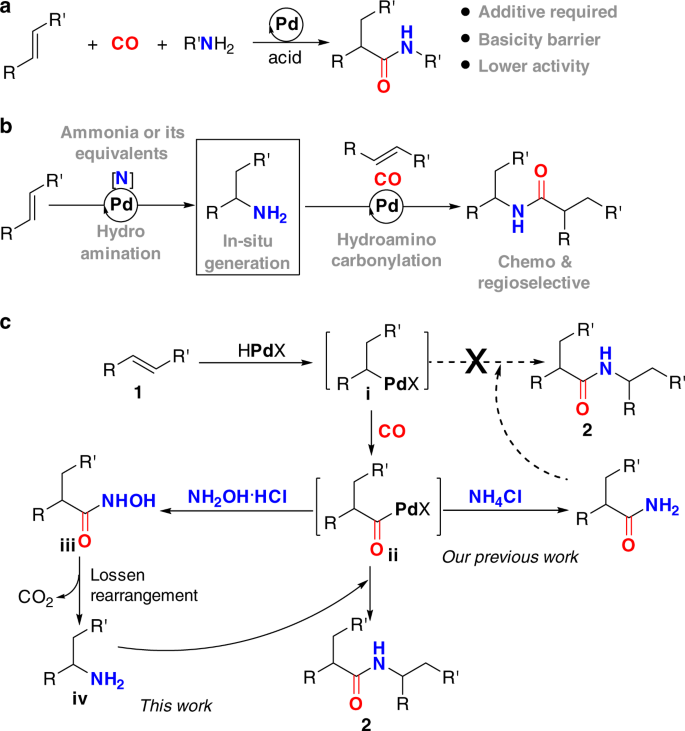
Palladium-catalyzed relay hydroaminocarbonylation of alkenes with hydroxylamine hydrochloride as an ammonia equivalent | Communications Chemistry